ORAL İLAÇLARIN OKÜLER YAN ETKİLERİ
The ocular adverse effects of oral drugs
- Rawan Ahmad, Hemal Mehta
- Aust Prescr 2021;44:129-36
- 2 August 2021
- DOI: 10.18773/austprescr.2021.028
SUMMARY
Some commonly prescribed drugs have ocular adverse effects. Many parts of the eye can be affected by oral drugs. Some ocular adverse effects may be reversed with medical or surgical intervention whereas other drugs may cause irreversible loss of vision.
The risk of visual loss can be reduced by a number of approaches, including monitoring for ocular toxicity, reducing the drug dose, or stopping the drug and looking for an alternative. This can be supported by good communication between the prescribing clinician and ophthalmologist.
Infrequent or delayed ocular adverse effects may not be identified in clinical trials of new drugs. Reporting adverse events is therefore important.
Introduction
Drugs that are taken orally are systemically absorbed, with the potential to affect all parts of the body including the eye. Its rich blood supply and relatively small mass increase the susceptibility of the eye to drugrelated adverse effects.1 Many parts of the eye can be affected by oral drugs. Patients presenting with unexplained ocular symptoms should be asked which drugs they are taking. Table 1 shows some of the ocular adverse effects to consider with common orally administered drug classes.235
Table 1 - Common or serious ocular adverse effects of selected oral drugs
|
Oral drug |
Ocular adverse effects |
Management |
Action required |
|
Alpha1 adrenergic receptor antagonists • tamsulosin • dutasteride • finasteride |
Intraoperative floppy iris syndrome resulting in the iris becoming mobile during cataract surgery.2 This increases the risk of iris damage with greater chance of postoperative blurred vision, sensitivity to light and difficulty driving at night. Floppy iris syndrome can also increase the risk of damage to the posterior lens capsule – a poor prognostic factor for cataract surgery. |
Usually there is no need to stop the drug as stopping it does not necessarily prevent floppy iris syndrome. The ophthalmic surgeon can take precautions during cataract surgery if aware the patient is on this class of drug. |
Inform ophthalmic surgeon of present or past drug use if patient is referred for cataract surgery. |
|
Antiarrhythmics • amiodarone |
Corneal microdeposits called vortex keratopathy have been reported in most patients. |
Most are asymptomatic and do not require intervention but advanced corneal deposits can cause visual symptoms.3 |
Routine ophthalmology referral only if patient is symptomatic. |
|
|
Amiodarone can rarely cause optic neuropathy which may result in permanent visual loss.3 |
Consider discussing this potential ocular adverse event when prescribing or dispensing. |
Immediate ophthalmology referral if patient has symptoms of optic neuropathy. |
|
• digoxin |
Ocular symptoms include yellowing of vision, scintillating scotoma and blurred vision. These changes are likely due to direct photoreceptor toxicity.4 |
The visual symptoms usually reverse when digoxin is stopped. |
Routine ophthalmology referral if patient is symptomatic. |
|
Allopurinol |
Long-term use of allopurinol has been associated with the development of cortical and subcapsular cataract formation.5 |
Consider discussing this potential ocular adverse event when prescribing or dispensing. |
Routine ophthalmology referral if patient is symptomatic. |
|
Anticholinergics including: • antihistamines • antipsychotics • antispasmodics, e.g. oxybutynin6 |
Reduced tear production (dry eyes). Dilated pupils.7 Decreased accommodation.7 Risk of acute angle-closure glaucoma in patients with narrow angles – this is unlikely to occur if the patient had previous cataract surgery.7 |
Consider discussing angle-closure glaucoma when prescribing or dispensing. |
Immediate ophthalmology referral if angle-closure glaucoma is suspected. Otherwise, routine ophthalmology referral if ocular symptoms persist after stopping the anticholinergic drug or if it has to continue.7 |
|
Anticoagulants • aspirin • clopidogrel • warfarin • apixaban • dabigatran • rivaroxaban • ticagrelor |
Potential haemorrhagic complications during peri-ocular surgery.8 |
Discussion of possible cessation before certain types of surgery such as eyelid surgery.8 Anticoagulants do not always need to be stopped before cataract surgery, especially if it is performed under topical anaesthesia. The ophthalmic surgeon should provide guidance. Anticoagulants are not routinely stopped before intravitreal therapy. |
Immediate ophthalmology referral if there is bleeding in the eye. Subconjunctival haemorrhage on the surface of the eye will usually resolve within 3 weeks and does not require ophthalmology referral if there are no other ocular symptoms. |
|
Antiepileptics • topiramate |
Topiramate can cause secondary angle-closure glaucoma hence onset can sometimes be delayed. Most cases present in the first few weeks of treatment although some cases are reported within hours of treatment.9-11 Visual field defects.9 Oculogyric crisis – a dystonic reaction characterised by prolonged involuntary upward deviation of the eyes.9,10 Uveitis.9 |
Bilateral angle closure should raise suspicions of a secondary angle-closure mechanism. Topical or systemic aqueous suppressants and topical cycloplegics (e.g. tropicamide, cyclopentolate or atropine) are initially used to manage secondary angle closure. The initial management of primary angle closure is different where, in addition to aqueous suppression, the pupils are constricted with pilocarpine. |
Immediate ophthalmology referral for symptomatic patients.9 |
|
• gabapentin |
Cases of nystagmus, diplopia and visual field defects have been reported.12 |
The incidence of visual field defects is low and therefore routine eye screening is not widely recommended. An annual computerised visual field test as part of the patient’s routine eye check with an optometrist or ophthalmologist is reasonable. |
Immediate ophthalmology referral is recommended for symptomatic patients. |
|
• vigabatrin |
Patients can develop visual field constriction attributable to vigabatrin. Patients may not notice visual field loss until the central field is affected.13 Patients can also develop optic atrophy with pallor of the optic nerve head.13 |
A baseline visual field should be obtained before treatment. Computerised visual field assessment should be repeated every 6 months for 5 years and can be extended to annually thereafter in patients who have no visual field defects. The visual field defects may not reverse on cessation of the drug but would likely worsen with continued use.13 |
Ensure patients on vigabatrin are referred for ophthalmology screening. |
|
Bisphosphonates • alendronate sodium • risedronate • zoledronic acid |
These drugs can cause inflammation in the eye leading to conjunctivitis, episcleritis, scleritis, keratitis or uveitis.14 Corneal and scleral melting have been reported. Symptoms usually emerge more slowly (usually 6–8 weeks) with oral versus intravenous dosing.14 |
Ocular signs usually abate after stopping the bisphosphonate but might require topical or oral corticosteroids.14 |
Corneal and scleral melting require urgent ophthalmology referral. Conjunctivitis and episcleritis can be referred routinely to ophthalmology. |
|
Chloroquine-based drugs • chloroquine • hydroxychloroquine |
Patients are usually asymptomatic early on but advanced maculopathy or peripheral retinopathy can result in irreversible visual loss.15-17 Patients who have been taking hydroxychloroquine for a period longer than 5 years or have been taking doses greater than 5 mg/kg/day are at an increased risk of maculopathy. Renal or liver impairment or concomitant tamoxifen use increase the risk.15-17 |
Ocular screening consists of structural imaging such as optical coherence tomography or fundus autofluorescence, and functional tests such as computerised visual fields or less widely available multifocal electroretinograms.15-17 Chloroquine has a worse adverse effect profile than hydroxychloroquine and all patients on chloroquine require baseline and at least annual ocular monitoring. |
Ensure patients on chloroquine-based drugs are referred for ophthalmology screening. |
|
Corticosteroids • prednisolone • dexamethasone |
Corticosteroid-induced raised intraocular pressure can lead to glaucoma.19 Open-angle glaucoma is asymptomatic until advanced. Corticosteroids can accelerate cataract progression and cause posterior subcapsular cataracts. |
Open-angle glaucoma is usually managed with drops or laser. However, more complicated cases may require surgery. Intraocular pressure should be monitored by an optometrist with referral to an ophthalmologist if it is raised. Intraocular pressure often returns to normal following cessation of corticosteroids. |
Routine ophthalmology referral if patient is symptomatic with cataract or noted to have raised intraocular pressure. If the intraocular pressure is greater than 30 mmHg the referral should be expedited. |
|
Ethambutol |
Ethambutol can cause optic neuropathy characterised by bilateral central visual loss, decreased colour vision, central visual field defects and eventually optic atrophy.20 Toxicity is dose-related with an incidence of 18% above 35 mg/kg/day and under 1% at 15 mg/kg/day or less. |
Between 30% and 64% of patients will show some visual recovery if the optic neuropathy is detected early and the ethambutol is stopped.20 Baseline ophthalmology assessment is required. Re-assessment every 3 months while the patient remains on low-dose treatment or every month if the dose is above 15 mg/kg/day. |
Ensure patients on ethambutol are referred for ophthalmology screening. |
|
Fingolimod |
Fingolimod-associated macular oedema has been reported in approximately 0.4% of patients. It usually develops within 4 months of starting treatment.21 Patients can report blurred vision, distortion and impaired reading vision. |
Ophthalmic assessment including optical coherence tomography imaging at baseline and then 3–4 months after starting treatment. Ocular surveillance can be annual thereafter or earlier if the patient becomes symptomatic. Fingolimod should ideally not be started within 3 months of intraocular surgery as it can make differentiating postoperative macula oedema from drug toxicity challenging. Patients with fingolimod-associated macular oedema do not always have to stop the treatment. Stopping may cause a rebound of multiple sclerosis. The macular oedema can often be treated with local therapy. |
Ensure patients on fingolimod are referred for ophthalmology screening. |
|
Isotretinoin and vitamin A |
Ocular surface signs:22 • blepharoconjunctivitis • chalazia • corneal opacities • dry eyes. Retinopathy:22 • excess vitamin A may worsen certain retinal dystrophies. |
Visual symptoms should stop with drug cessation if not too advanced.22 |
Routine ophthalmology referral if symptomatic. |
|
MEK inhibitors e.g. crizotinib |
Visual disturbances have been reported in patients taking oral MEK inhibitors. Reported ocular adverse effects include: • decreased visual acuity • visual field defects • dry-eye symptoms • eyelid abnormalities23 • retinal vein occlusion • MEK-associated retinopathy.23 |
MEK-associated retinopathy has been reported to improve after drug cessation.23 |
Routine ophthalmology referral if symptomatic. |
|
Pentosan polysulfate |
Pentosan polysulfate maculopathy has been reported to have an incidence of 16%. Retinal pigment epithelial lesions are more common with prolonged use and higher daily and cumulative doses.24 |
Multimodal retinal imaging can identify pentosan polysulfate maculopathy before irreversible macular damage has developed. Patients with cumulative dosages over 500 g should receive annual ophthalmology assessment and those with cumulative dosages over 1000 g, especially over 1500 g, should be monitored for macular toxicity even more regularly. |
Ensure patients on pentosan polysulfate with a cumulative dose over 500 g are referred for ophthalmology screening. |
|
Phenothiazines |
High doses result in abnormal pigmentation of the eyelids, conjunctiva and cornea.25 High doses have been reported to cause corneal epithelial changes.25 A rare but serious adverse effect is development of corneal oedema. |
Epithelial keratopathy does not usually cause any visual impairment and normally clears after stopping the drug. Corneal oedema may result in irreversible visual impairment if the drug is not stopped promptly.25 |
Immediate ophthalmology referral in symptomatic patients. |
|
Phosphodiesterase type 5 inhibitors • sildenafil • tadalafil |
A bluish discoloration of vision may occur 1–2 hours after ingestion.26 Persistent blurred vision has been reported secondary to non-arteritic ischaemic optic neuropathy, cilioretinal artery occlusion, or central serous chorioretinopathy.27,28 |
Consider discussing this potential ocular adverse event when prescribing or dispensing. |
Routine ophthalmology referral if any persisting visual symptoms.27,28 |
|
Tamoxifen |
Intraretinal crystalline deposits, macular oedema and punctate retinal pigmentary changes have been reported.29-32 The degree of toxicity is related to the dose and duration of tamoxifen use.30 |
Systematic screening of all symptom-free patients taking the lower dose (20–40 mg a day) for metastatic breast cancer has not been shown to be of high yield in detecting ocular toxity.31 However, routine ophthalmological assessment is reasonable in asymptomatic patients on higher doses, treatment for more than 5 years or pre-existing macular disease.32 Early crystalline maculopathy without visual symptoms does not always require stopping the drug. Alternative oncological therapies are available if serious ocular complications arise. |
Baseline ophthalmology assessment and regular monitoring in high-risk groups. Immediate ophthalmology referral in symptomatic patients. |
|
Tetracyclines • doxycycline • tetracycline |
Nausea, vomiting and morning headaches may be symptoms of idiopathic intracranial hypertension which can lead to permanent loss of vision.33 |
Consider discussing the symptoms of idiopathic intracranial hypertension when prescribing or dispensing this medication. |
Urgent ophthalmology referral if patient is symptomatic. |
|
Thiazolidinediones (glitazones) • pioglitazone • rosiglitazone |
May result in development of macular oedema.34,35 |
Consider an alternative drug in patients with diabetic retinopathy.34,35 |
Diabetic retinopathy screening as per national guidelines and immediate ophthalmic referral in symptomatic patients. |
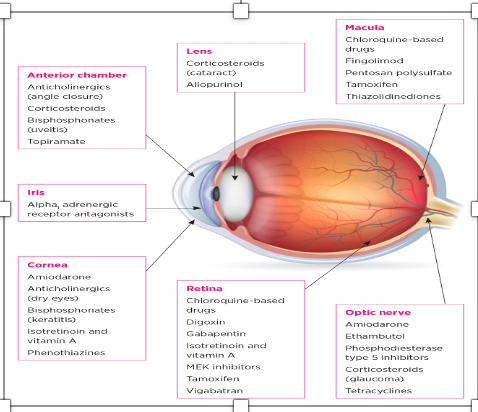
Structures of the eye affected by oral drugs
Drugs may cause symptoms characteristic of specific eye diseases (Table 2). Some drugs, such as those with anticholinergic activity, affect several parts of the eye (see Fig.).
Table 2 - Symptoms associated with disease of different parts of the eye
|
Disease |
Symptoms |
|
Angleclosure glaucoma |
Ocular pain, haloes around lights, nausea and vomiting |
|
Corneal disease |
Photophobia, grittiness, ocular pain, blurred vision |
|
Ocular inflammation (uveitis, scleritis) |
Photophobia, blurred vision, ocular pain, floaters |
|
Cataract |
Constant clouded, blurred and dim vision, glare (for example when driving) |
|
Macular disease |
Distortion, central scotoma, blurred vision, difficulty reading |
|
Optic neuropathy |
Impaired colour vision, red desaturation, blurred vision, field defect |
Fig. - Examples of drugs affecting different parts of the eye
Anterior chamber and cornea
Anticholinergic drugs cause the relaxation of the ciliary muscle, leading to temporary blurred vision.7 They can contribute to dryeye symptoms by suppressing normal parasympathetic activity. Anticholinergic drugs can also cause the severe adverse effect of angleclosure glaucoma.7 This usually occurs in longsighted patients with narrow drainage angles. Angle closure is highly unlikely in patients who have had cataract surgery because removing the lens deepens the anterior chamber.
Bisphosphonates can cause inflammation leading to conjunctivitis, episcleritis, scleritis, keratitis and uveitis. The exact mechanism of this ocular inflammation is not yet known.14 Symptoms usually emerge more slowly (usually 6–8 weeks) with oral versus intravenous dosing. Unilateral and bilateral ocular presentations have been reported.14 Bisphosphonates may also cause corneal or scleral melting which requires urgent ophthalmology referral.
Amiodarone, and other drugs such as hydroxychloroquine, can deposit on the basal epithelial layer of the cornea causing the formation of whirllike corneal microdeposits called vortex keratopathy.3 This is usually asymptomatic and does not require stopping the treatment. However, advanced corneal deposits can cause visual symptoms, hence patients should be referred for an ophthalmic review if the keratopathy affects their vision.
Phenothiazines can cause the development of corneal epithelial changes that can eventually result in corneal oedema. The changes of corneal oedema can become permanent if the drug is not stopped promptly.25
The longterm use of corticosteroids via any route of administration may increase intraocular pressure by interfering with the outflow from the trabecular meshwork. This is a significant risk factor for the development of glaucoma.19
Iris and lens
Corticosteroids can accelerate cataract progression. Classically, they cause posterior subcapsular cataracts which develop more rapidly than typical agerelated nuclear sclerotic cataracts.19 This may relate to corticosteroidinduced changes to gene transcription in the epithelial cells of the lens.19 The longterm use of allopurinol has also been linked to cataract formation.5
The use of alpha1 adrenergic receptor antagonists, such as tamsulosin, may lead to the iris becoming mobile during cataract surgery, a phenomenon called intraoperative floppy iris syndrome.2 The mechanism is probably related to the blockade of alpha1 adrenergic receptors within the dilator muscle of the iris. Floppy iris syndrome may increase the likelihood of iris or posterior capsule damage during intraocular surgery. Usually there is no need to stop the drug as stopping it does not necessarily prevent floppy iris syndrome. Instead, the ophthalmic surgeon should be informed so they can take appropriate precautions during cataract surgery.
Retina
Chloroquine and hydroxychloroquine can cause degeneration of the retina and retinal pigment epithelium.1517,36 The risk of toxicity is increased with higher doses and a longer duration of treatment. Additional risk factors are renal or liver impairment or concomitant tamoxifen use. Toxicity can lead to reduced visual acuity, paracentral scotomas and bull’s eye (parafoveal) maculopathy. Retinopathy does not always develop in a bull’s eye pattern as a more peripheral paracentral pattern of damage can be observed in patients of Asian backgrounds. As a result, screening practices need to be adjusted to recognise both paracentral and parafoveal retinopathy. The damage may be irreversible. Ocular screening during treatment is therefore recommended (Table 1).1517,36,37
Tamoxifen retinal toxicity can cause symptoms of decreased visual acuity and colour vision with signs of intraretinal crystalline deposits, macular oedema and punctate retinal pigment epithelial changes. These adverse effects usually occur with higher doses of the tamoxifen (Table 1).2932
Digoxin can cause ocular symptoms including yellowing of vision, scintillating scotoma and blurred vision. These changes are likely to be due to direct photoreceptor toxicity.4 The visual symptoms usually reverse when digoxin is discontinued.
Fingolimod, used in the management of multiple sclerosis, has secondary effects on the function of the vascular–endothelial barrier, thereby potentially compromising the blood–retina barrier. Fingolimod associated macular oedema can cause blurred vision, distortion and impaired reading vision.21 Patients with fingolimodassociated macular oedema do not always have to stop treatment because of the risk of a flareup of the multiple sclerosis. The macular oedema can often be treated with ocular therapy.
New ocular adverse effects are being identified with the increased use of oral immunebased therapies such as kinase inhibitors. These include visual disturbances, visual field defects as well as retinal vein occlusion and MEKassociated retinopathy. Communication between the physician and ophthalmologist is important if ocular adverse effects are suspected.23
Thiazolidinediones, such as pioglitazone, have been associated with systemic fluid retention. These drugs can worsen diabetic macular oedema, especially in patients with preexisting diabetic retinopathy.6,34,35
Drugs for erectile dysfunction, such as sildenafil, can inhibit photoreceptor function. This may cause transient blurring of vision or altered colour perception. There have also been reports of nonarteritic ischaemic optic neuropathy, cilioretinal artery occlusion and central serous chorioretinopathy.2628 Routine referral to an ophthalmologist is required if there are persisting visual symptoms.
Central serous chorioretinopathy is characterised by the accumulation of fluid in the central vision of patients. Symptoms include blurred central vision, distortion and washing out of colours. Central serous chorioretinopathy is associated with systemic steroid use and has been reported with sildenafil.
Vigabatrin has been associated with the development of visual field constriction. Patients may not notice any visual field loss until the central field is affected. The visual field defects do not reverse when the drug is stopped and may worsen with continued use. Hence, a computerised visual field assessment is usually obtained before treatment and is repeated every six months for five years. This can then be extended to an annual review if the patient does not have any visual field defects.13
Optic nerve
Amiodarone may rarely induce optic neuropathy.3 This is characterised by swelling of the optic discs in addition to the typical symptoms of optic neuropathy (Table 2). The main differential diagnosis is nonarteritic anterior ischaemic optic neuropathy, which is more common in patients with vasculopathy and is associated with an altitudinal monocular visual field defect (superior or inferior half of the vision is affected).
Tetracyclines have been reported to cause idiopathic intracranial hypertension which in some instances can lead to permanent loss of vision.33 Nausea, vomiting and morning headaches, as well as the symptoms of optic neuropathy (Table 2), can be suggestive of idiopathic intracranial hypertension.
Ethambutol can cause optic neuropathy. Animal studies have suggested retinal ganglion cells are predominantly affected.32 Risk factors include higher doses, prolonged use, poor renal function and concurrent antiretroviral therapy.
Management of ocular adverse effects
Consultation with an ophthalmologist is recommended if a drug is suspected to be affecting a patient’s vision. Interventions can include screening before treatment, monitoring for ocular toxicity, reducing drug doses, or stopping the drug and looking for an alternative. Some ocular adverse effects such as raised intraocular pressure can be managed with medical or laser therapy. Cataracts can be managed with surgical intervention. However, some ocular adverse events such as macular atrophy can cause irreversible visual loss, hence the need to screen for damage at an early stage.
Pharmacovigilance
Medicine is a constantly evolving field with new drugs being developed all the time. Many ocular adverse effects are reported during the clinical trials of drug development but others emerge later. Postmarketing surveillance, such as the Black Triangle Scheme, has proved to be valuable in the identification of rare and otherwise not previously reported adverse effects. It is important to keep an open mind when prescribing new drugs and be vigilant in assessing any possible ocular adverse effects. Adverse events should be reported to the Therapeutic Goods Administration.
Conclusion
Commonly used oral drugs can cause ocular adverse effects. As well as retinal toxicity, oral drugs can affect other parts of the eye including the cornea, lens and optic nerve. Consider drugs as a possible cause of unexplained ocular symptoms. Communication between the prescribing clinician and ophthalmologist will facilitate the best possible patient care.
Conflicts of interest: none declared
This article is peer-reviewed.
Australian Prescriber welcomes Feedback.
References
- Santaella RM, Fraunfelder FW. Ocular adverse effects associated with systemic medications : recognition and management. Drugs 2007;67:7593.
- Fung A, McCluskey P. Tamsulosininduced intraoperative floppy iris syndrome during cataract surgery. Aust Prescr 2010;33:889.
- Nagra PK, Foroozan R, Savino PJ, Castillo I, Sergott RC. Amiodarone induced optic neuropathy. Br J Ophthalmol 2003;87:4202.
- Renard D, Rubli E, Voide N, Borruat FX, Rothuizen LE. Spectrum of digoxininduced ocular toxicity: a case report and literature review. BMC Res Notes 2015;8:368.
- Luo C, Chen X, Jin H, Yao K. The association between gout and cataract risk: A metaanalysis. PLoS One 2017;12:e0180188.
- Yang MC, Lin KY. Druginduced acute angleclosure glaucoma: a review. J Curr Glaucoma Pract 2019;13:1049.
- Sekeroglu MA, Hekimoglu E, Anayol MA, Tasci Y, Dolen I. An overlooked effect of systemic anticholinergics: alteration on accommodation amplitude. Int J Ophthalmol 2016;9:7435.
- Shieh WS, Sridhar J, Hong BK, Maguire JI, Rahimy E, Shahlaee A, et al. Ophthalmic complications associated with direct oral anticoagulant medications. Semin Ophthalmol 2017;32:6149.
- Hesami O, Hosseini SS, Kazemi N, HosseiniZijoud SM, Moghaddam NB, Assarzadegan F, et al. Evaluation of ocular side effects in the patients on topiramate therapy for control of migrainous headache. J Clin Diagn Res 2016;10:NC0104.
- Abtahi MA, Abtahi SH, Fazel F, Roomizadeh P, Etemadifar M, Jenab K, et al. Topiramate and the vision: a systematic review. Clin Ophthalmol 2012;6:11731.
- Craig JE, Ong TJ, Louis DL, Wells JM. Mechanism of topiramateinduced acuteonset myopia and angle closure glaucoma. Am J Ophthalmol 2004;137:1935.
- Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017;9:1321.
- Hawker MJ, Astbury NJ. The ocular side effects of vigabatrin (Sabril): information and guidance for screening. Eye (Lond) 2008;22:10978.
- McKague M, Jorgenson D, Buxton KA. Ocular side effects of bisphosphonates: A case report and literature review. Can Fam Physician 2010;56:10157.
- Grierson DJ. Hydroxychloroquine and visual screening in a rheumatology outpatient clinic. Ann Rheum Dis 1997;56:18890.
- Browning DJ. Hydroxychloroquine and chloroquine retinopathy: screening for drug toxicity. Am J Ophthalmol 2002;133:64956.
- Michaelides M, Stover NB, Francis PJ, Weleber RG. Retinal toxicity associated with hydroxychloroquine and chloroquine: risk factors, screening, and progression despite cessation of therapy. Arch Ophthalmol 2011;129:309.
- Mavrikakis I, Sfikakis PP, Mavrikakis E, Rougas K, Nikolaou A, Kostopoulos C, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology 2003;110:13216.
- Jones R 3rd, Rhee DJ. Corticosteroidinduced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol 2006;17:1637.
- Chamberlain PD, Sadaka A, Berry S, Lee AG. Ethambutol optic neuropathy. Curr Opin Ophthalmol 2017;28:54551.
- Jain N, Bhatti MT. Macular edema associated with fingolimod. EyeNet Magazine May 2021. [cited 2021 Jul 1]
- Fraunfelder FT, Fraunfelder FW, Edwards R. Ocular side effects possibly associated with isotretinoin usage. Am J Ophthalmol 2001;132:299305.
- MéndezMartínez S, Calvo P, RuizMoreno O, Pardiñas Barón N, Leciñena Bueno J, Gil Ruiz MD, et al. Ocular adverse events associated with MEK inhibitors. Retina 2019;39:143550.
- Wang D, Velaga SB, Grondin C, Au A, Nittala M, Chhablani J, et al. Pentosan polysulfate maculopathy: prevalence, spectrum of disease, and choroidal imaging analysis based on prospective screening. Am J Ophthalmol 2021;227:12538.
- Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents: a review. CNS Drugs 2010;24:50126.
- Moschos MM, Nitoda E. Pathophysiology of visual disorders induced by phosphodiesterase inhibitors in the treatment of erectile dysfunction. Drug Des Devel Ther 2016;10:340713.
- Hayreh SS. Erectile dysfunction drugs and nonarteritic anterior ischemic optic neuropathy: is there a cause and effect relationship? J Neuroophthalmol 2005;25:2958.
- Karaarslan C. Ocular side effects of sildenafil that persist beyond 24 h – a case series. Front Neurol 2020;11:67.
- Alwitry A, Gardner I. Tamoxifen maculopathy. Arch Ophthalmol 2002;120:1402.
- Nayfield SG, Gorin MB. Tamoxifenassociated eye disease. A review. J Clin Oncol 1996;14:101826.
- Heier JS, Dragoo RA, Enzenauer RW, Waterhouse WJ. Screening for ocular toxicity in asymptomatic patients treated with tamoxifen. Am J Ophthalmol 1994;117:7725.
- Therssen R, Jansen E, Leys A, Rutten J, Meyskens J. Screening for tamoxifen ocular toxicity: a prospective study. Eur J Ophthalmol 1995;5:2304.
- Digre KB. Not so benign intracranial hypertension. BMJ 2003;326:6134.
- Idris I, Warren G, Donnelly R. Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes. Arch Intern Med 2012;172:100511.
- Ryan EH Jr, Han DP, Ramsay RC, Cantrill HL, Bennett SR, Dev S, et al. Diabetic macular edema associated with glitazone use. Retina 2006;26:56270.
- Royal College of Ophthalmologists. Hydroxychloroquine and chloroquine retinopathy: recommendations on monitoring 2020. Updated 2020 Dec 16. [cited 2021 Jul 1]
- Royal Australian and New Zealand College of Ophthalmologists. Guidelines for screening for hydroxychloroquine retinopathy. Sydney: RANZCO; 2015. [cited 2021 Jul 1]







